Chemical properties of germanium - Health effects of Germanium - Environmental effects of germanium
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
More from 'Elements'
Lenntech (European Head Office)
- Question: Part A What Is Wrong With The Notation 1s22s22p63s23p63d104s24p2 For Germanium (atomic Number 32)? The 4s Subshell Should Appear Before The 3d Subshell. The 3p Subshell Should Have More Than 6 Electrons. The 3d Subshell Can Hold Only 8 Electrons.
- Chemical properties, health and environmental effects of germanium. Electronegativity according to Pauling.
- Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard-brittle, grayish-white metalloid in the carbon group, chemically similar to its group neighbors silicon and tin. Pure germanium is a semiconductor with an appearance similar to elemental silicon.
- Germanium is an element with atomic symbol Ge, atomic number 32, and atomic weight 72.64.
Germanium's existence was predicted before anyone isolated it. This was a triumph for Dmitri Mendeleev in his construction of the periodic table. By 1869, Mendeleev had assembled a crude table of the known elements, arranging them according to their chemical properties and atomic weights. But his table had a number of prominent gaps.

Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
Configuration Of Germanium Atomic Number
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com

Germanium Atomic Number
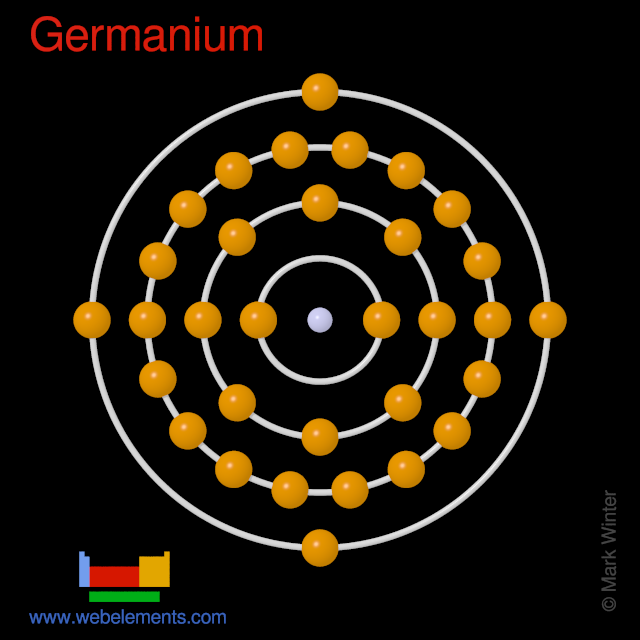
Germanium Atomic Number Electron Configuration
Lenntech DMCC (Middle East)
Gallium Atomic Number And Mass
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Germanium Atomic Number Symbol
Germanium Atomic Number
Copyright © 1998-2021 Lenntech B.V. All rights reserved
